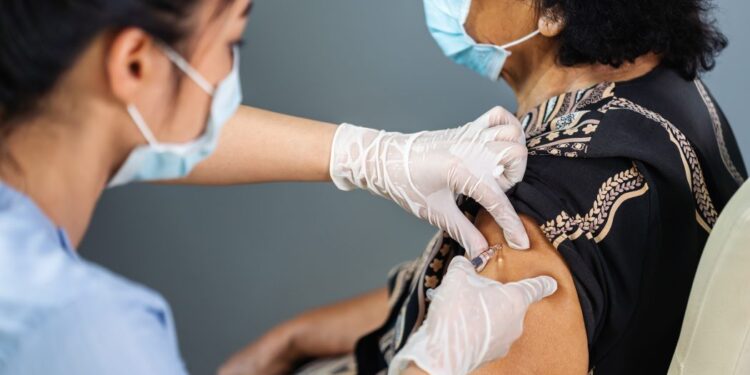
The FDA approved fourth Moderna and Pfizer vaccine doses for everyone over 50 as China lock-downed financial center China earlier this week due to a surge of contagions.
The authorizations falls under the category expanded US emergency use authorization for vulnerable individuals in particular individuals 12 years or older with compromised immune systems and people over 50.
The FDA made its decision based on an ongoing open label study of 154 healthcare workers 18 and older in a single center in Israel. According to Pfizer its vaccine declines in effectiveness against COVID-19 after 3 to 6 months, implying that an additional booster can improve protection against severe disease and death due to the virus.
Possible side effects of the vaccine are severe allergic reaction, inflammation of the heart muscle and injection site pain.