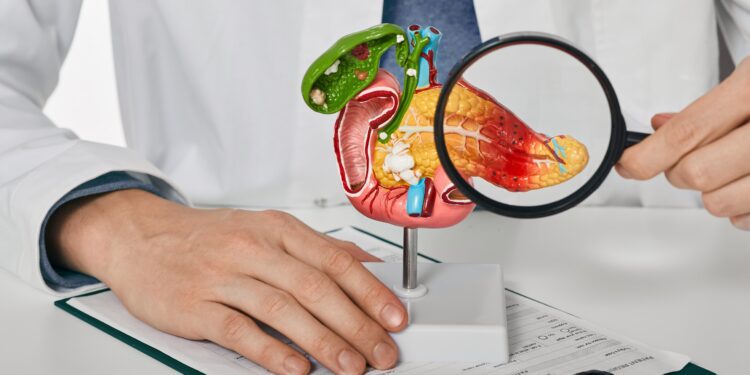
The FDA has approved Ipsen’s Onivyde as part of a multidrug treatment for patients with newly diagnosed metastatic pancreatic cancer. The combo, Nalirifox, is the first new treatment in more than 10 years indicated for newly diagnosed pancreatic cancer. Onivyde, which Ipsen bought in 2017, had already been approved for people with previously treated pancreatic cancer.
Analyst expect Onivyde salesto hit around $284 million in 2025, compared with around $176 million last year. A company spokesperson estimates sales could peak at $535 million. Ipsen has said it aims to increase sales by at least 7% each year from last year through 2027 at constant exchange rates.
Onivyde has a patent cliff in late 2027, so Ipsen will also look to deals to continue its growth. The company had about $2 billion to dedicate to deals at the end of last year.
“Our journey will be driven by the combination of the growth platforms, our new medicines and more external-innovation transactions to come,” Ipsen CEO David Loew has said.