A realistic assessment of the heroic effort to administer billions of doses of COVD-19 vaccines to an agonized global population is necessary. The stakes could not be higher, says McKinsey.
The COVID-19 vaccines of the BioNTech and Pfizer partnership (Pfizer–BioNTech) and Moderna have received Emergency Use Authorization in Canada, the European Union, the United Kingdom, the United States, and other countries. Many frontline workers and priority population segments have received their first doses. Vaccines from AstraZeneca, Johnson & Johnson, and several other global manufacturers also are arriving and being distributed for administration around the world. This monumental global effort has shattered the record for vaccine development: the fastest previous vaccine project, Merck’s mumps vaccine, was four and a half years in development (1963–67).
In certain places, the COVID-19-vaccine effort has hit a few speed bumps; stockpiles have accumulated, and deployment to vulnerable countries and at-risk groups has been slower than expected. Nonetheless, experts have expressed confidence that safe and highly efficacious vaccines are reaching the market, and we are beginning to see “the light at the end of the tunnel” of this devastating pandemic. The epidemiological end to the COVID-19 pandemic seemed like an optimistic dream a few short months ago, but, with the development, approval, and rollout of several vaccines, it is now practically realizable in much of the world.
To arrive at the postpandemic era, in which populations experience herd immunity, vast numbers of dedicated individuals will need to continue working intensely in the months and years ahead. In this article, we consider elements of this enormous undertaking, the risks that are inherent, and potential means of further accelerating vaccination.
A common operating model of COVID-19-vaccine delivery, shown in the interactive, demonstrates the complexity of the task at hand. Essentially, the interactive is a qualitative risk map, showing the many stages of vaccine deployment and highlighting areas of potential failure as one party interacts with another. A breakdown at any point in the deployment process can set off a cascade, shutting down the entire system.
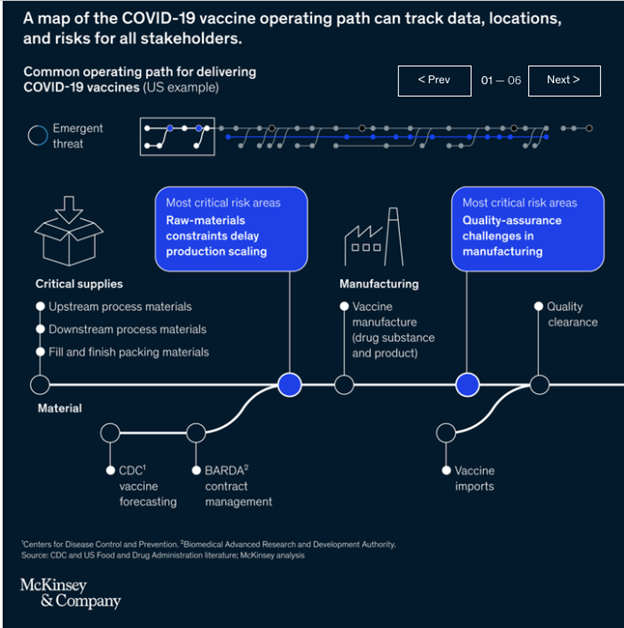

In the United States alone, hundreds of organizations play a role in vaccine deployment, adapting their operations to meet demands for volume, speed, and better technology. Suppliers, manufacturers, and regulators are collaborating to ramp up production of vaccines. Massive volumes handled, distributed, and stored through cold chains must adhere to safety regulations. Tens of thousands of transporters, vaccine handlers, medical and pharmacy staff, and frontline workers have required training on the specific characteristics of each manufacturer’s distinct vaccines.
At the receiving end, vulnerable populations—especially in developing countries—could face added hurdles, including difficulty in reaching administrative sites, getting time off from work to receive doses, and arranging childcare for the same. Historical wariness of interacting with authorities can also be a barrier. A further issue is vaccine skepticism, affecting a certain segment of all populations, including the United States.
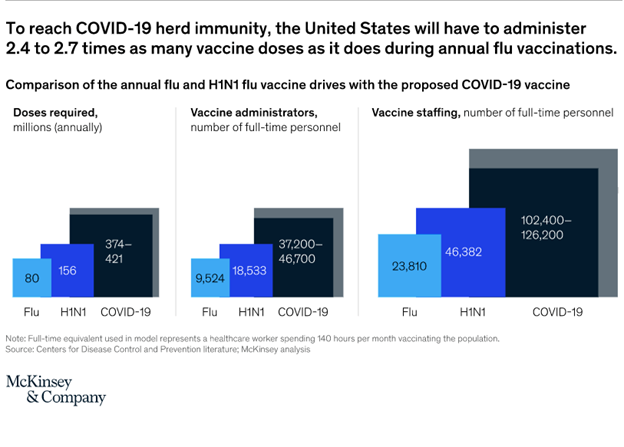
In the United States and other countries with sufficient vaccine quantities and adoption, herd immunity by October 2021 is conceivable, but for that to happen, more than twice as many doses of COVID-19 vaccines will have to be administered each month as were administered during the 2009 H1N1 flu vaccine drive. Cumulatively, between 2.4 and 2.7 times as many doses (mainly because of the double-dose requirement) will be needed as are used for the annual flu vaccinations (exhibit).

Scientists, doctors, other healthcare workers, clinical-trial participants, and regulators have been working intensely to develop, distribute, and administer the vaccines that will help end this pandemic. Their often-heroic efforts give cause for hope. An array of complex challenges are still before us, as production is ramped up and rollouts are planned and executed. A realistic risk assessment of vaccine deployment is needed, because future success cannot be taken for granted. Much needs to be done for the promise of COVID-19 vaccines to become a reality.
Critical emerging risks
The common operating model provides the details of end-to-end vaccine deployment. From this model, we have highlighted six possible areas of risk to the rapid delivery of COVID-19 vaccines in the United States and elsewhere. In a later section, we outline possible approaches to mitigating each of these risks, with practical steps for organizations that have a role in vaccine deployment.
Read the full article at https://www.mckinsey.com/business-functions/risk/our-insights/the-risks-and-challenges-of-the-global-covid-19-vaccine-rollout
This article is a collaborative effort by the global Risk Practice and authors from across the firm, including Gaurav Agrawal, Tara Azimi, Jennifer Heller, Pooja Kumar, Mihir Mysore, Parag Patel, Adam Sabow, Shubham Singhal, and Joseph Truesdale.