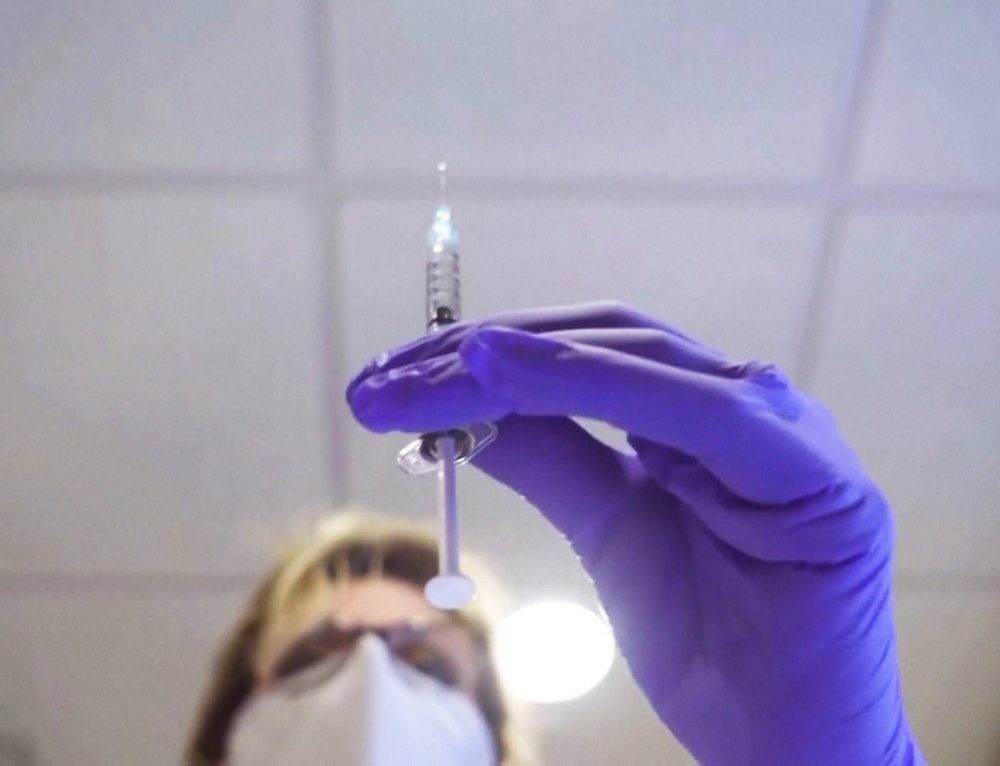
A COVID vaccine developed between the US and Germany may be the good news we have been waiting for, yet plans to deliver raises questions about logistics.
American pharmaceutical giant Pfizer and Germany’s BioNTech said Monday their coronavirus vaccine was more than 90% effective in preventing Covid-19 among those without evidence of prior infection, hailing the announcement as “a great day for science and humanity.”
“I think we can see light at the end of the tunnel,” Pfizer Chairman and CEO Dr. Albert Bourla said in an interview with CNBC. “I believe this is likely the most significant medical advance in the last 100 years, if you count the impact this will have in public health, global economy.”
The coronavirus pandemic has taken over 1.2 million lives worldwide and scientists are hoping for a vaccine that is at least 75% effective. White House coronavirus advisor Dr. Anthony Fauci has said even one that is 50% or 60% effective would be acceptable.
U.S. stock futures soared following the news. Futures on the Dow Jones Industrial Average surged 1,646 points, implying an opening gain of more than 1,630 points.
Pfizer’s results are based on the first interim efficacy analysis conducted by an external and independent Data Monitoring Committee. The analysis evaluated 94 confirmed Covid-19 infections among the trial’s 43,538 participants. Pfizer and BioNTech said the case split between vaccinated individuals and those who received a placebo indicated a vaccine efficacy rate of above 90% at seven days after the second dose.
The vaccine could be available in limited use as early as late December and widely available by the third quarter of 2021. Its efficacy percentage may vary, however, as safety and additional data continue to be collected.
“With today’s news, we are a significant step closer to providing people around the world with a much-needed breakthrough to help bring an end to this global health crisis,” Bourla said in a statement. “We look forward to sharing additional efficacy and safety data generated from thousands of participants in the coming weeks.”
Based on current projections, Pfizer and BioNTech expect to produce up to 50 million vaccine doses in 2020, and up to 1.3 billion doses in 2021. In July, the companies reached a nearly $2 billion agreement with the U.S. government to supply 100 million doses.
Questions about logistics
The announcement by Pfizer and BioNTech is, of course, incredibly positive, yet plans to deliver hundreds of millions of coronavirus vaccines around the world raises questions about logistics and distribution in part because of the need to store and transport them in supercooled containers.
Pfizer’s vaccine requires a storage temperature of minus 94 degrees Fahrenheit. By comparison, Moderna has said its vaccine can be stored at minus 4 degrees Fahrenheit.
The company reportedly plans to distribute these suitcase-sized boxes from hubs in Kalamzoo, Michigan, and Puurs, Belgium, onto as many as two dozen trucks per day, allowing for the daily transit of roughly 7.6 million doses to nearby airports.
The vaccine may be widely available within 12 to 18 months since Chinese scientists first identified the coronavirus—a record-breaking time frame for a process that normally takes about a decade for an effective and safe vaccine.